If you’ve studied chemistry even briefly, chances are you’ve seen a question that begins with the line “a solution of KCl is saturated at 50 °C.” It looks simple, but this single sentence carries a lot of hidden meaning. For many students, this is where confusion starts, because understanding saturation, temperature, and solubility all at once isn’t always straightforward.
This article breaks everything down in a clear, human way, without sounding like a textbook. We’ll focus on meaning first, then gradually move into calculations, numerical problems, and common exam-style variations, using all the relevant concepts naturally.
A Solution of KCl Is Saturated at 50 °C
When a question says a solution of KCl is saturated at 50 °C, it’s not just giving background information. It is telling you something very specific about how much potassium chloride is dissolved in water at that temperature.
A KCl solution saturated at 50 °C means:
- The solution contains the maximum possible amount of KCl that can dissolve in water at 50 degree Celsius
- Any additional KCl added at this temperature will not dissolve
- The solution is in equilibrium, dissolution and crystallization are happening at the same rate
This is why phrases like:
- kcl solution saturated at 50 c
- saturated solution of kcl at 50 c
- potassium chloride solution saturated at 50 c
are all essentially describing the same chemical condition.
What Does a Saturated Solution Mean?
Before jumping into numbers, it’s important to answer a basic question students often ask: what does saturated solution mean?
A saturated solution is one in which:
- The solvent (usually water) has dissolved as much solute as it can at a given temperature
- Extra solute remains undissolved
- The system has reached a balance between dissolving and crystallizing
In the case of KCl:
- KCl is the solute
- Water is the solvent
- Temperature controls how much KCl can dissolve
This idea forms the concept of saturation in solutions, and temperature plays a central role in it.
What Is a Saturated Solution of KCl at 50 °C?
A saturated solution of KCl at 50 °C means that water has dissolved the maximum possible amount of potassium chloride at that exact temperature, no more, no less.
This also explains the phrase “meaning of saturated solution at 50 c.” The temperature is not optional information. If the temperature changes, the saturation point changes too.
So when a question says kcl saturated solution at 50 c, you should immediately think:
- Solubility data
- Solubility tables or curves
- Grams of KCl per 100 g of water
Why Temperature Matters So Much in KCl Solutions
One of the most searched ideas around this topic is how saturation depends on temperature. That’s because most ionic solids, including KCl, become more soluble as temperature increases.
For potassium chloride:
- As temperature rises, KCl solubility increases
- As temperature drops, excess KCl crystallizes out
This explains why:
- effect of temperature on solubility of kcl
- why temperature affects solubility of kcl
are such common search queries.
In short, temperature controls how much KCl water can “hold.”
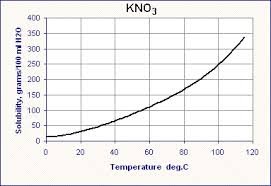
Solubility of KCl at 50 °C
Now let’s move to the most important numerical idea: solubility of KCl at 50 °C.
From standard solubility data:
- At 50 °C, about 42–43 grams of KCl dissolve in 100 g of water
This value may vary slightly depending on the table used, but exam questions usually specify or expect this approximate number.
This answers searches like:
- grams of kcl dissolved at 50 c
- solubility of potassium chloride at 50 °c
- solubility data of kcl
Whenever you see a solution of KCl is saturated at 50 °C, you can safely assume that this amount of KCl is already dissolved.
Understanding the Solubility Curve of KCl
The solubility curve of KCl visually shows how solubility changes with temperature. At 50 °C:
- The curve is higher than at room temperature
- This confirms that more KCl dissolves at higher temperatures
Students who understand the curve can answer questions without memorizing values, especially conceptual ones like:
- What happens when temperature increases?
- What happens when the solution cools?
How Many Grams of KCl Are Dissolved at 50 °C?
This is one of the most direct exam questions:
How many grams of solute are dissolved in 100 g of water if a solution of KCl is saturated at 50 °C?
The logic is simple:
- A saturated solution already contains the maximum solute
- From solubility data, that amount is about 43 g
So the answer directly comes from solubility tables, not guesswork.
This type of question supports searches like:
- calculation based on kcl saturated solution
- how to calculate mass of kcl in saturated solution
Composition of a Saturated KCl Solution
When students are asked about composition of saturated solution of KCl, they’re really being asked about proportions.
At 50 °C:
- Solute = ~43 g KCl
- Solvent = 100 g water
- Total mass of solution = ~143 g
This also explains mass of solute and solvent in saturated solution, which is often a stepping stone to percentage calculations.
Concentration and Percentage Composition of KCl Solution
Another common extension is finding the percentage composition of KCl solution.
Mass percent formula:
- (Mass of solute / Mass of solution) × 100
Using typical values:
- (43 / 143) × 100 ≈ 30%
This directly answers:
- concentration of saturated kcl solution
- saturated solution concentration calculation
Numerical Problems Based on Saturated KCl Solution
Most numerical on KCl saturated solution questions follow a predictable structure:
- Given temperature
- Given saturation condition
- Asked for mass, concentration, or change on cooling/heating
That’s why chemistry numericals on solubility appear so often in exams for:
- Class 9
- Class 10
- Class 11
What Happens When a Saturated KCl Solution Is Cooled?
When a saturated solution of KCl at 50 °C is cooled, its ability to hold solute decreases. The solution becomes supersaturated temporarily, and excess KCl starts to crystallize out. This process is a direct application of the temperature dependence of solubility.
Key points to remember:
- The lower the temperature, the less KCl can stay dissolved.
- Crystals form until the solution reaches new equilibrium at the cooler temperature.
- This concept is critical for solving questions like:
- kcl solution cooled from 50 c to 30 c
- crystals formed from saturated kcl solution
- precipitation from saturated solution
- kcl solution cooled from 50 c to 30 c
Understanding this process is important for both conceptual reasoning and numerical problems in exams.
Calculating the Amount of KCl Crystallized on Cooling
Once you know how much KCl dissolves at 50 °C and the solubility at a lower temperature, calculating the amount of crystallized KCl is straightforward.
Steps:
- Determine the mass of KCl initially dissolved at 50 °C (typically ~43 g per 100 g of water).
- Check the solubility at the lower temperature (for example, 30 g per 100 g of water at 30 °C).
- Subtract the lower temperature solubility from the initial solubility:
- Crystallized KCl = 43 g − 30 g = 13 g
- Crystallized KCl = 43 g − 30 g = 13 g
This method addresses problems based on saturated solution of kcl and how to solve questions on saturated kcl solution in a very practical way.
Saturated vs Unsaturated KCl Solutions
Students often confuse saturated and unsaturated solutions, which can lead to mistakes in numerical or conceptual questions.
- Saturated solution: contains the maximum solute at a given temperature. Any extra solute will not dissolve.
- Unsaturated solution: contains less than the maximum solute, meaning more KCl can dissolve if added.
This distinction is crucial in questions asking about adding extra solute:
- what happens when more kcl is added to saturated solution
Remember, for KCl at 50 °C:
- Saturated = ~43 g per 100 g water
- Unsaturated = any amount below this threshold
Why Exam Questions Emphasize Saturation at 50 °C
Board exams and class tests frequently use this scenario because it tests multiple core concepts at once:
- Understanding of saturation and solubility
- Ability to read and use solubility tables
- Skill in calculating concentrations, mass, and crystallization
- Grasping effect of temperature on solubility
Searches like kcl saturated solution class 9 and kcl solubility questions class 11 appear because this topic spans multiple grades.
Common Mistakes Students Make
Even though the problem seems simple, students often trip up due to:
- Ignoring temperature: assuming solubility is constant at any temperature.
- Adding extra solute: thinking it will dissolve in a saturated solution.
- Mixing mass of solute with total solution mass incorrectly when calculating percentage composition.
- Forgetting crystallization on cooling, which is key for numerical answers.
Being aware of these pitfalls helps when tackling questions like:
- how do you know a kcl solution is saturated
- why kcl solution becomes saturated at 50 c
How to Approach Any Question That Mentions “Saturated at 50 °C”
Here’s a simple step-by-step approach to solve these questions confidently:
- Identify the given temperature and check solubility tables for KCl at that temperature.
- Determine the mass of KCl dissolved (per 100 g of water, usually).
- Look for changes like heating or cooling that affect solubility.
- Calculate crystallization or additional solute needed if required.
- Double-check mass percent and total solution mass if asked.
By following these steps, you cover numerical problems, conceptual reasoning, and exam traps all in one go.
Practical Example: Solving a KCl Saturation Problem
Problem Statement:
A solution of KCl is saturated at 50 °C. The solubility of KCl at 50 °C is 43 g per 100 g of water. Calculate:
- The total mass of the solution
- Mass percent of KCl in the solution
- Mass of KCl crystallized if the solution is cooled to 30 °C, where solubility is 34 g per 100 g water
Solution Step-by-Step:
- Total mass of solution:
- Solute = 43 g, Solvent = 100 g
- Total = 143 g
- Solute = 43 g, Solvent = 100 g
- Mass percent of KCl:
- (43 / 143) × 100 ≈ 30%
- (43 / 143) × 100 ≈ 30%
- Crystallization on cooling:
- KCl that can stay dissolved at 30 °C = 34 g
- Crystallized KCl = 43 − 34 = 9 g
- KCl that can stay dissolved at 30 °C = 34 g
This simple framework can be applied to all exam-style problems involving KCl or other salts.
Key Takeaways from KCl Saturation Problems
Even without stating a “conclusion,” here are the conceptual lessons students learn from these questions:
- Saturation depends on both solute and temperature
- Crystallization occurs on cooling, and solute mass calculations are straightforward with solubility tables
- Understanding the difference between saturated and unsaturated solutions is essential
- Percentage composition, total solution mass, and solute-solvent ratio are key calculations
- Step-by-step logical thinking beats memorization in board exams